|
|
马上注册,结交更多好友,享用更多功能,让你轻松玩转社区。
您需要 登录 才可以下载或查看,没有账号?注册

×
18 October 2007
Researchers in the US have taken a snapshot of the inside of a gold nanoparticle, shedding crucial new light on one of chemistry's longest-standing questions: how does sulfur bind to gold?1
For centuries it has been known that gold, one of the most inert of metals, will react with sulfur and that sulfur-containing compounds will bind to gold. Theories abound about how this can occur but to date there has been little solid evidence to support any of them.
Now a team led by Robert Kornberg from Stanford University in California has solved the atomic structure of gold nanoparticles coated with the sulfur-containing compound p-mercaptobenzoic acid, revealing the nature of the bond in unprecedented detail.
'Gold atoms are zero valent and in general do not want to participate in bonding to other atoms,' said Robert Whetten of the Georgia Institute of Technology in Atlanta, co-author of a commentary on the work.2 'For years people have speculated about the nature of the bonding between gold and sulfur, but we have had to wait until now for the photograph.'
Kornberg's group successfully prepared gold nanoparticles of a uniform size - something that has so far proved elusive and which itself represents a key breakthrough as it confirms gold nanoparticles have a well-defined structure, rather than simply being random colloidal agglomerations of atoms.
The team then resolved the geometry and internal structure of the nanoparticles using x-ray crystallography and showed that each nanoparticle contains 102 gold atoms, 23 of which bind to thiol sulfurs of acid molecules.
But exactly how these gold-sulfur complexes bond to the metallic gold beneath is unclear. The bond distance is longer than that between other gold atoms in the nanoparticle, suggesting a comparitively weak interaction that is still strong enough to prevent detachment.
The nanoparticles also proved to be chiral - mirror images of each other.
The structure disproves certain earlier ideas of the gold-thiol interface and suggests a partially covalent character to the bonding. 'This structure is easily accessible to density-functional theory calculations, which could establish the stability of the proposed sulfur binding configuration,' Robinson added. 'It is to be hoped that such a calculation will be attempted very soon.'
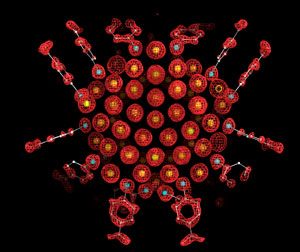
X-ray crystal structure of the gold nanoparticle in this study, which consists of 102 gold atoms and 44 p-mercaptobenzoic acids. This image shows and electron density map (the red mesh) and the atomic structure, with the gold atoms depicted as yellow spheres
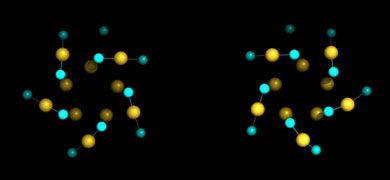
The nanoparticles proved to be chiral, composed of substructures that are mirror images of each other.
References
1 P D Jadzinsky et al., Science, 2007, 318, 430 (DOI: 10.1126/science.1148624)
2 R L Whetten & R C Price, Science, 2007, 318, 407 (DOI: 10.1126/science.1142441) |
|